Hydrolysis of the extracellular
signalling substance ATP by 5'-nucleotidase
Biological background
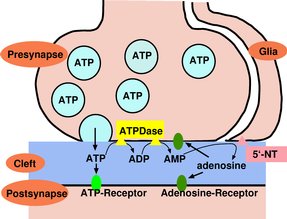 |
5'-Nucleotidase (5'-NT) from E. coli is a zinc-containing enzyme,
which is also known as UDP-sugar hydrolase. The periplasmic enzyme is probably
involved in the hydrolysis of external UDP-glucose and of nucleotides,
for utilization by the cell.
Animal ecto-5'-nucleotidases are related to this bacterial enzyme.
Ecto-nucleotidases are important for the formation and hydrolysis of nucleosides
and nucleotides as extracellular signaling substances. Animal 5'-NTs function
together with other ecto-nucleotidases in the termination of the neurotransmitter
action of ATP in the brain and periphery. |
Protein structure
|
|
We have crystallised the enzyme and determined the protein structure
with the help of the MAD and MIR methods. The best crystals diffracted
to 1.7 Angstrom resolution.
The enzyme consists of a larger N-terminal domain (green) and a smaller
C-terminal domain (blue). The two domains are linked by a long alpha-helix.
Shown here is the structure of the closed (active) conformation. The catalytic
center, which contains two zinc ions, is located at the interface between
the two domains. |
Substrate binding
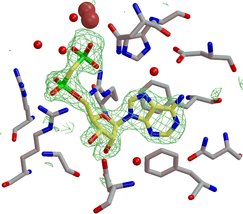 |
Cocrystal structures with inhibitors and products define the
substrate specificity pocket and provide important information to understand
the enzyme function. The inhibitor shown to the left (yellow carbon atoms)
is a non-hydrolyzable analog of ADP, because the scissile anhydride bond
between the phosphate groups is replaced by a methylene group. The enzyme
was cocrystallized with the inhibitor, which indeed did bind to the catalytic
site. Shown in green is the electron density map, obtained from the X-ray
experiment, which defines the binding mode of the inhibitor. |
|



