Junior Research Group of Dr. Anette Kaiser

New address from 01/2023:
University of Leipzig Medical Center (UKL), Department of Anesthesiology and Intensive Care
Liebigstr. 19
D-04103 Leipzig
PHD Students

Master Students

Research Projects
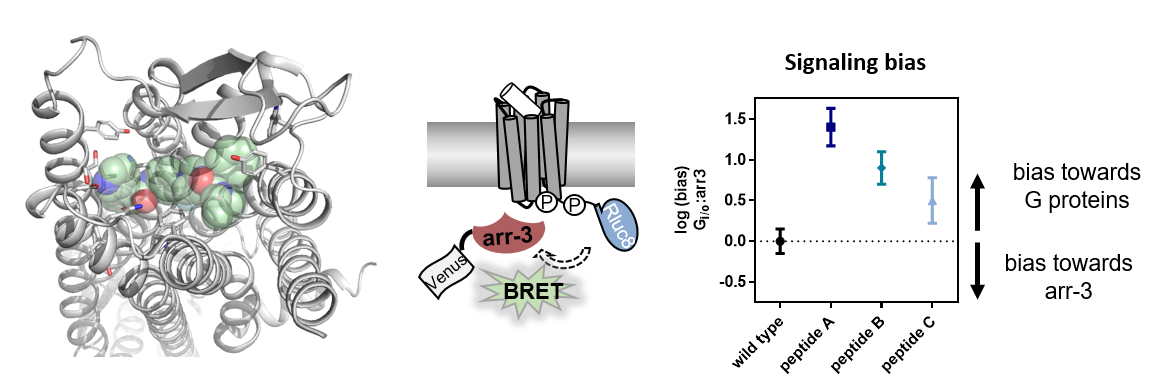
Molecular control of G protein-coupled receptor signaling
G protein-coupled receptors (GPCRs) are the largest class of transmembrane proteins with a huge variety of ligands. They have essential functions in health and disease, and are the molecular target for about one third of all current pharmaceuticals. Peptide- and protein-activated GPCRs are still underrepresented in the clinical intervention, but the number of peptide therapeutics is constantly increasing. Understanding how peptide ligands interact to their receptors, and which pathways are triggered downstream of receptor activation is an essential step toward the development of more potent and selective drugs. To address these questions, I use rational mutagenesis of receptor and ligands guided by structural models and measure multiple downstream pathways in cellulo. Moreover, protein-protein interactions are analyzed in cellular contexts to obtain spatio-temporal insights into receptor signaling.
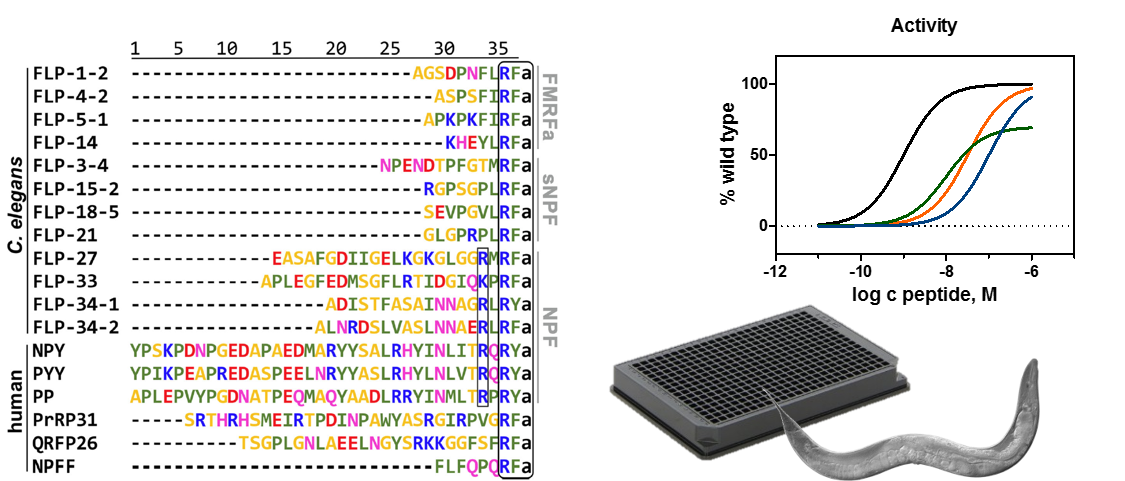
Evolution of neuropeptide GPCR specificity and signaling
Peptide-binding GPCRs are involved in the regulation of energy homeostasis and neuroendocrine regulation. Reflecting their essential physiologic functions, many GPCR systems are conserved down to basal animals. This deep evolutional conservation also opens avenues to study common and individual principles of receptor function. Moreover, relatively simple model organism might be used to study aspects of receptor signaling or test compounds in an in vivo context. The round worm C. elegans is a valuable model organism in this regard, as all main G protein pathways and an arrestin homolog are present. Moreover, it offers a vast genetic toolbox and its translucence enables fluorescent techniques. We have previously demonstrated homology of the C. elegans NPR system to the mammalian NPY family and plan to extend these studies within the consortium of the SFB1423.
Publications
A complete list af publications can be found via PubMed.
Most important publications:
| Tang T, Tan Q, Han S, Diemar A, Löbner K, Wang H, Schüß C, Behr V, Mörl K, Wang M, Chu X, Yi C, Keller M, Kofoed J, Reedtz-Runge S, Kaiser A#, Beck-Sickinger AG#, Zhao Q#, Wu B#. Receptor-specific recognition of NPY peptides revealed by structures of NPY receptors. Sci Adv. 2022;8(18):eabm1232. | Abstract |
| Groß VE, Gershkovich MM, Schöneberg T, Kaiser A#, Prömel S#. NanoBRET in C. elegans illuminates functional receptor interactions in real time. BMC Mol Cell Biol. 2022;23(1):8. | Abstract |
| Gershkovich MM, Groß VE, Vu O, Schoeder CT, Meiler J, Prömel S, Kaiser A#. Structural Perspective on Ancient Neuropeptide Y-like System reveals Hallmark Features for Peptide Recognition and Receptor Activation. J Mol Biol. 2021;433(13):166992. PMID: 33865871; | Abstract |
| Kaiser A*, Wanka L*, Ziffert I, Beck-Sickinger AG. Biased agonists at the human Y1 receptor lead to prolonged membrane residency and extended receptor G protein interaction. Cell Mol Life Sci. 2020;77(22):4675-4691. | Abstract |
| Gershkovich MM, Groß VE, Kaiser A#, Prömel S#. Pharmacological and functional similarities of the human neuropeptide Y system in C. elegans challenges phylogenetic views on the FLP/NPR system. Cell Commun Signal. 2019;17(1):123. | Abstract |
| Kaiser A#, Hempel C, Wanka L, Schubert M, Hamm HE, Beck-Sickinger AG. G Protein Preassembly Rescues Efficacy of W6.48 Toggle Mutations in Neuropeptide Y2 Receptor. Mol Pharmacol. 2018;93(4):387-401. | Abstract |


