Development of advanced imaging techniques
Engineered fusion proteins of signalling proteins intramolecularly linked to various color variants of the green fluorescent protein have fueled numerous approaches to clarify spatiotemporal properties of signal transduction in living cells. Fluorescent fusion proteins are visible without additional cofactors and serve to follow the localization of signaling proteins in living cells at the subcellular level and in a sub-second temporal resolution.
To improve the detection of weak changes in fluorescent fusion proteins, especially near the plasma membrane we developed a microscopic device that combines the technique of total internal reflection fluorescence microscopy (TIRFM) with other techniques like FRAP, FRET, and FLIM. TIRFM is the method of choice to selectively excite fluorescent molecules in the vicinity of the plasma membrane. With a penetration depth of 100-150 nm, the evanescent wave excitation restricts the z-axis to a volume that is smaller than that of CLSM and most other fluorescence-based live cell imaging methods.
Fluorescence Redistribution after Photobleaching (FRAP) is used to determine mobility parameters of fluorescent molecules. We developed a microscopic setup that enables prismless (trough the lens) TIR illumination of samples in combination with spot photobleaching, which allows the detection of minute mobility changes of weakly effective and short-lived interactions with the plasma membrane.
Fluorescence Resonance Energy Transfer (FRET) is used to detect the spatial proximity of differenlty labeled molecules with a sub-nanometer resolution. In combination with TIRFM, interactions at the plasma membrane can be selectively monitored.
The fluorescence lifetime of a fluorophor depends on its physico-chemical environment. It is measured by exciting the sample with short laser pulses and measuring the fluorescence decay by time-correlated single photon counting (TCSPC). Fluorescence lifetime analysis in living cells is used to determine the local environment of the fluorescent probe, i.e. pH or hydrophobicity. Moreover, fluorescence lifetime imaging (FLIM) can be used to measure FRET since the lifetime of the excited state of a donor fluorochrome is shortened in the presence of a closely positioned and spectrally suitable FRET acceptor. We are also able to measure dynamic anisotropy of molecules using the TCSPC method, which is especially useful to detect homo-FRET.
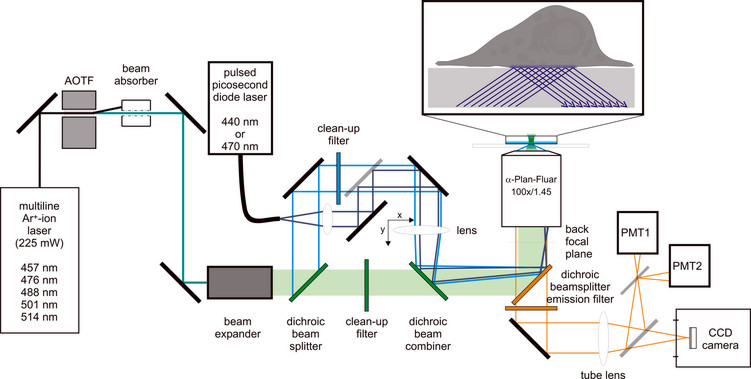
Schematic illustration of the beampath used for FRAP (green), TIR illumination (cyan), and pulsed picosecond TIR excitation (dark blue).