

Institute of Bioanalytical Chemistry
Prof. Dr. Norbert Sträter
 |
 |
| Center
for Biotechnology and Biomedicine Institute of Bioanalytical Chemistry |
|
| Structural
analysis of
biopolymers Prof. Dr. Norbert Sträter |
|
Ecto-5'-Nucleotidase (CD73): Generation of extracellular adenosine from AMP
|
| Ecto-5'-Nucleotidase (e5NT), also known as CD73, is part of the purinergic signaling cascade in that it hydrolyzes AMP to adenosine. Thereby, adenosine signaling via P1 receptors is switched on. E5NT is involved in the development of chronic pain, hypoxia and inflammation. Furthermore, CD73 has been shown to be overexpressed in many cancer types for tumor promotion and metastasis. CD73 inhibitors are of interest for cancer immunotherapy. We expressed e5NT in E. coli without the C-terminal GPI signal sequence which naturally anchors the enzyme to the cell membrane. The protein was refolded from inclusion bodies, purified and finally crystallized. E5NT shows a similar structure as its bacterial homolog: the two-domain enzyme contains the dimetal center in the N-terminal domain (blue and orange) and the substrate binding pocket in the C-terminal domain (green and yellow). However, e5NT adopts a dimeric arrangment via interaction of the C-terminal domains [3]. Furthermore, e5NT was crystallized in open and closed conformations which indicate domain rotations of up to 114°. |
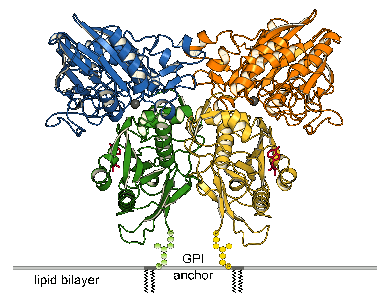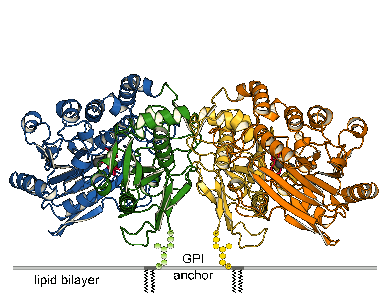 Domain motion of the dimer of e5NT |
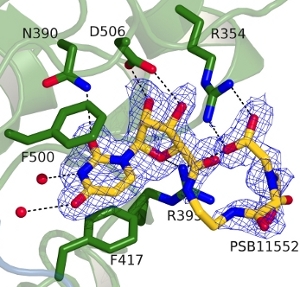 Binding mode of PSB11552 to the substrate binding pocket of e5NT |
E5NT is specific for the hydrolysis of AMP (whereas E. coli 5NT
hydrolyzes ATP, ADP and AMP). From the current data we hypothesize that the domain motion
controls the substrate specificity of e5NT. Future work aims to further our understanding of the exclusive specificity of e5NT towards AMP which distinguishes the mammalian enzyme from the bacterial homologs. Furthermore, due to its role in the development of cancer and other diseases, e5NT is an important target in structure based drug design. We aim to find lead structures to contribute to the development of pharmaceuticals. |
|
Coworkers Karen KnappMatthias Zebisch Jan Pippel Emma R. Scaletti Collaborations Professor Dr. Christa Müller, Universität Bonn, Pharmazeutisches Institut Publications
|