

Institute of Bioanalytical Chemistry
Prof. Dr. Norbert Sträter
 |
 |
| Center
for Biotechnology and Biomedicine Institute of Bioanalytical Chemistry |
|
|
Structural
analysis of
biopolymers Prof. Dr. Norbert Sträter |
|
NTPDases from microbial pathogens |
| Nucleotides
released as extracellular signaling molecules are hydrolyzed by
nucleoside triphosphate diphosphohydrolases (NTPDases).
These enzymes are found in mammals as well as in microbial pathogens where they are thought to modulate the host immune response to an infection by hydrolysis of host ATP. The enzyme also plays a role in purine salvage. Toxoplasma gondii, an intracellular protozoan responsible for the disease toxoplasmosis, expresses two NTPDases which are related to the virulence of the parasite. Interestingly, T. gondii NTPDases differ from other NTPDases in that 1) they are homotetramers, 2) they require reductive activation, and 3) despite 97% sequence identity they differ largely in their ability to hydrolyze ATP and ADP. We characterized the activation mechanism by protein crystallography and enzyme kinetics [1,3]. Furthermore, it was shown that the altered catalysis of ATP and ADP by the two T. gondii NTPDase isoforms is dependent on amino acids 492 and 493 which are part of the base binding site. These residues produce two alternative binding modes of the nucleotide base [5]. |
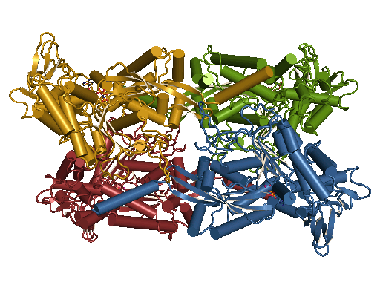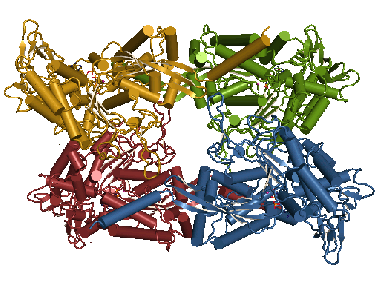 T. gondii NTPDases form homotetramers. After reduction of the disulfide C258/C268 a domain motion is induced. This enables the closure of the active site cleft in each monomer and thereby catalysis of ATP and ADP. This animation shows these conformational changes. |
L. pneumophila NTPDase1 was crystallized with several transition-state mimics which allow a detailed description of the catalytic course. |
Legionella pneumophila,
the causative agent of Legionnaire's disease, expresses one NTPDase.
This LpNTPDase1 is required for the multiplication in the replicative
vacuole. We produced LpNTPDase1 in high yields from a bacterial expression system to employ this enzyme as a model system for structural and functional characterization of NTPDases. By crystallization of LpNTPDase1 in several crystal forms with nonhydrolyzable substrate analogs it was possible to get detailed insight into the phosphoryl transfer of NTPDases. Furthermore, we obtained different open and closed conformations which further our understanding of the flexibility of NTPDases and of a substrate induced domain closure motion [2,4]. |