

Institute of Bioanalytical Chemistry
Prof. Dr. Norbert Sträter
 |
 |
| Center
for Biotechnology and Biomedicine Institute of Bioanalytical Chemistry |
|
|
Structural
analysis of
biopolymers Prof. Dr. Norbert Sträter |
|
Purinergic Signalling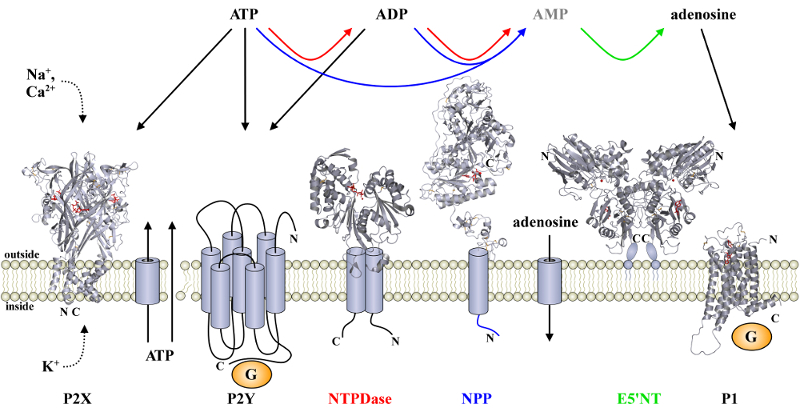 In addition to its
important
cellular function in metabolism, ATP also serves as an extracellular
signalling substance. It is released by exocytosis or via transporters
to the extracellular space and it acts on P2X receptors, which are
ligand-gated ion channels, or on the P2Y receptors, which are
G-protein coupled receptors. These signalling pathways via ATP and
other nucleotides are termed purinergic signalling. Extracellular
nucleotides influence a wide variety of short-term (acute) physiological
processes, including exocrine and endocrine secretion, immune responses,
inflammation, nociceptive mechanosensory transduction, platelet aggregation and
endothelial-mediated vasodilatation. Long-term
(trophic) processes affected are cell proliferation, differentiation, migration
and death as for example in development, regeneration and cancer.
As for any signalling molecule, its action has to be terminated with time. A number of extracelular nucleotidases are involved in the hydrolysis of the nucleotides. The NTPDases (ecto-nucleoside triphosphate diphosphohydrolases) dephosphorylate ATP via ADP to AMP. There are eight different types (NTPDase1-8) in humans. ADP also acts on specific receptors, whereas no receptor is currently known for AMP. 5-nucleotidase (5'-NT) catalyses the hydrolysis of AMP to adenosine. In addition to the receptors for extracellular nucleotides and nucleosides, also the ecto-nucleotidases have been recognized as pharmaceutical targets to interfere with purinergic signaling pathways. Specific inhibitors of NTPDases would not only be valuable tools in biopharmaceutical fundamental research. They would also constitute potential clinical therapeutics (e.g., in the treatment of chronic pain, immune system diseases and cancer) as they would prolong the physiological effects of extracellular nucleotides or simultaneously administered nucleotide analogs. With the aim to characterize its catalytic mechanism and in order to rationally develop specific inhibitors for pharmaceutical and biological studies, we have overexpressed the ectodomains of rat NTPDases 1 to 3 in E. coli and characterized the kinetic parameters in comparison to the full-length wild-type enzymes [Zebisch & Sträter, 2007]. M. Zebisch, N. Sträter Characterisation of rat NTPDase1, -2 and -3 ectodomains refolded from bacterial inclusion bodies Biochemistry 2007, 46, 11945-11956 M. Zebisch, N. Sträter Structural basis of signal conversion and inactivation by NTPDase2 in purinergic signalling Proc. Natl. Acad. Sci. USA 2008, 105, 6882-6887 |