Short Report by Gustavo Araiza: Yeast display of an H7-200 mutant library against H3 HK68 (August-November)
Influenza is a viral disease that results in the deaths of 650,000 people on a global scale every year. Immunocompromised populations are the most vulnerable to influenza infections, but treatment with therapeutic antibodies can improve patient outcomes. To this end, I have been attempting to redesign H7-200, a therapeutic antibody that only binds and protects against H7, towards the H3 subtype of influenza hemagglutinin. The binding site of H7-200, called the TI-II, is unique to H7 has not yet been documented with any other known subtypes. Thus, finding a TI-II-specific antibody that binds H3 would enable the development of more robust antibody cocktails for influenza treatments. My previous attempts to redesign H7-200 involved computational design and very low throughput experimental validation; just ten out of potentially thousands of sequences. To improve my chances of discovering an H3-binding H7-200 homolog, I designed and implemented two H7-200 mutant libraries for use in yeast display.
During my time in Leipzig University, I ran many experiments in pursuit of discovering a TI-II-specific, H3 binding homolog of H7-200. Our control data was solid and indicated that H7-200 could express at high levels as a surface-displayed protein, and demonstrated binding with its native binding partner, H7, while showing no off-target binding to H3. The mutant libraries, however, were more problematic. Initial attempts to express the H7-200 mutants on the surfaces of yeast cells were not as successful. We used a C-terminal Myc tag to detect successful surface expression, but found a very low cell density for the naïve libraries. Given some quality-control data, we narrowed the problem down to the DNA used to assemble the H7-200 mutant library, and that most of the DNA that entered the yeast was frame-shifted, resulting in low numbers of positive yeast clones. Most of my time was spent trying to fix this issue, and the majority of my work revolved around using various PCR-based techniques to eliminate frameshifted DNA. This was unfortunately not successful, and in the end we opted to isolate Myc-positive yeast cells from those not expressing H7-200 mutants. Thus far, we have run one more round of yeast display with H3 and H7. We saw some enrichment against both H3 and H7, though H7 has shown more hits. Clara’s lab will be continuing the yeast display until we have observed enough enrichment to send for sequencing. From here, I will evaluate the biochemical and biophysical properties of the successful hits at Vanderbilt University.
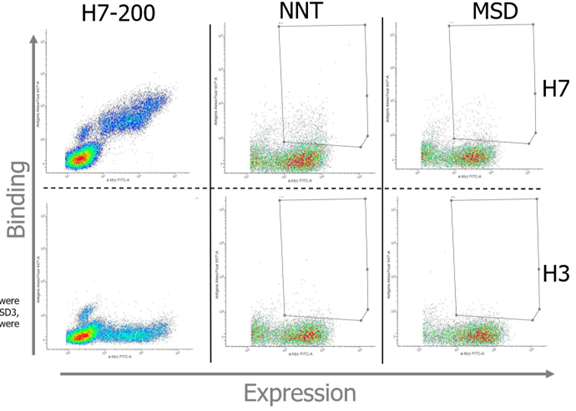
Figure 1. Flow cytometry of the first round of yeast surface-displayed wild-type H7-200 (left), random library (NNT, middle) and the multi-state design-guided library (MSD, right) against H7 SH13 (top row) and H3 HK68 (bottom row). Yeast pools were incubated with 1 µg/mL antigen for wild-type H7-200 and 10 µg/mL antigen for each library. Cell sorting was performed only on the libraries per the gates drawn on each plot. Thus far, the random library outperforms the computational library in terms of H3 binding, though the specificity of positive clones remains unclear.
Prior to this exchange, I had never been to Europe. I am incredibly grateful for this experience, not just for the research, but because I was able to experience a completely unique lifestyle from the one I have lived in the US. Furthermore, I made some really fantastic new friends in Clara Schoeder’s lab and so many memories that I will look fondly on well after my time in Leipzig. In Germany, I have/will visit Dresden, Munich, and Augsburg. I attended a conference in Copenhagen, Denmark, and will soon visit Prague, Czechia, and Vienna, Austria.

