A8 – Chemoenzymatic Synthesis of Oligohyaluronans with defined Sulfation Patterns for Binding Studies and the Generation of artificial extracellular Matrices
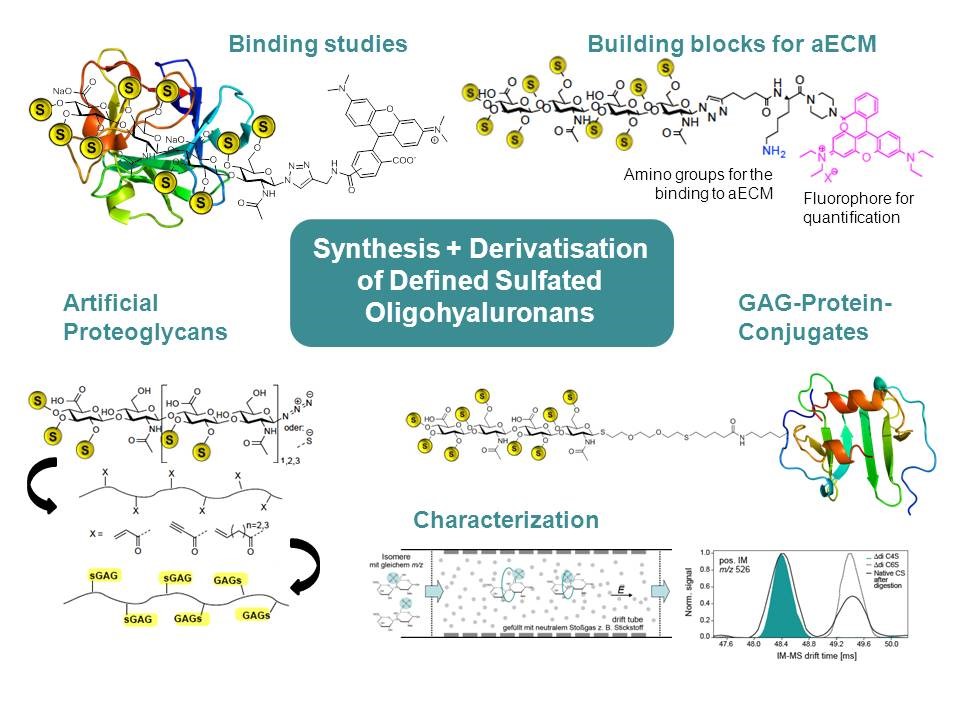
It is well known from previous studies that polysulfated glycosaminoglycans (GAG) are able to stimulate wound healing processes by their selective binding to cytokines and growth factors. During the new funding period of the Transregio we will investigate structure activity relationships of sulfated oligohyaluronans (oligo-HA) as ligands for mediators and growth factors as well as building blocks of the aECM in detail.
The synthesis of sulfated oligo-HA was successfully established by using chemoenzymatic methods. Using this approach some hyaluronan tetra-, hexa-, and octasaccharides with defined sulfation patterns could be already synthesized. The selective protection and ligation of the reducing anomeric end led to different fluorescence- and spin-labeled HA conjugates which were used for binding assays, structural characterization, immobilization on surfaces, and for functional biological studies. The obtained activity profiles will now be used for the development of specific, custom-made protein ligands. Finally, these optimized protein ligands will be used to synthesize rationally designed biomaterials (particularly GAG with defined sulfation patterns). Artificial but comparably small proteoglycans and sulfated GAG-conjugated proteins will be developed as multivalent assemblies of GAG with defined sulfation patterns.
The successful multi-dimensional mass spectrometric (MS) characterization of polysulfated compounds is aggravated by the lability of the sulfate ester linkage (and to a minor extent also by the glycosidic linkages). We have already shown with weakly sulfated reference compounds that ion mobility MS is an excellent tool to characterized isomeric carbohydrate sulfates. Subsequent to careful tuning of the instrument parameters the detection of such compounds can be achieved without major fragmentation. This work will be now extended for polysulfated compounds. Finally, we will compare these data with PFG-(pulsed field gradient)-NMR measurements. This is important because no fragmentation will be induced under conditions of NMR and the investigations can be performed in aqueous solution not in the gas phase.
Publications
- Schiller J, Lemmnitzer K, Dürig JN, Rademann J. Insights into structure, affinity, specificity, and function of GAG-protein interactions through the chemoenzymatic preparation of defined sulfated oligohyaluronans. Biological Chemistry, vol. 402, no. 11, 2021, pp. 1375-1384. https://doi.org/10.1515/hsz-2021-0165.
- Schnabelrauch M, Schiller J, Möller S, Scharnweber D, Hintze V. Chemically modified glycosaminoglycan derivatives as building blocks for biomaterial coatings and hydrogels. Biological Chemistry, vol. 402, no. 11, 2021, pp. 1385-1395. https://doi.org/10.1515/hsz-2021-0171.
- Großkopf H, Vogel S, Müller CD, Köhling S, Dürig JN, Möller S, Schnabelrauch M, Rademann J, Hempel U, von Bergen M, Schubert K. Identification of intracellular glycosaminoglycan-interacting proteins by affinity purification mass spectrometry. Biological Chemistry, vol. 402, no. 11, 2021, pp. 1427-1440. https://doi.org/10.1515/hsz-2021-0167.
- Balamurugan K, Koehler L, Dürig JN, Hempel U, Rademann J, Hintze V, Pisabarro MT. Structural insights into the modulation of PDGF/PDGFR-β complexation by hyaluronan derivatives. Biological Chemistry, vol. 402, no. 11, 2021, pp. 1441-1452. https://doi.org/10.1515/hsz-2021-0173.
- Kühn S, Freyse J, Atallah P, Rademann J, Freudenberg U, Werner C. Tuning the network charge of biohybrid hydrogel matrices to modulate the release of SDF-1. Biological Chemistry, vol. 402, no. 11, 2021, pp. 1453-1464. https://doi.org/10.1515/hsz-2021-0175.
- Clauder F, Möller S, Köhling S, Bellmann-Sickert K, Rademann J, Schnabelrauch M, Beck-Sickinger A G. Peptide-mediated surface coatings for the release of wound-healing cytokines. J Tissue Eng Regen Med 2020, September 6. DOI:10.1002/term.3123.
- Koehler L, Ruiz-Gómez G, Balamurugan K, Rother S, Freyse J, Möller S, Schnabelrauch M, Köhling S, Djordjevic S, Scharnweber D, Rademann J, Pisabarro MT, Hintze V. Dual Action of Sulfated Hyaluronan on Angiogenic Processes in Relation to Vascular Endothelial Growth Factor-A. Sci Rep. 2019 Dec 2;9(1):18143. doi: 10.1038/s41598-019-54211-0.
- Thönes S, Rother S, Wippold T, Blaszkiewicz J, Balamurugan K, Moeller S, Ruiz-Gómez G, Schnabelrauch M, Scharnweber D, Saalbach A, Rademann J, Pisabarro MT, Hintze V, Anderegg U. Hyaluronan/collagen hydrogels containing sulfated hyaluronan improve wound healing by sustained release of heparin-binding EGF-like growth factor. Acta Biomater. 2019 Mar 1;86:135-147. doi: 10.1016/j.actbio.2019.01.029 . Epub 2019 Jan 17.
- Koehler L, Samsonov S, Rother S, Vogel S, Köhling S, Moeller S, Schnabelrauch M, Rademann J, Hempel U, Pisabarro MT, Scharnweber D, Hintze V. Sulfated hyaluronan derivatives modulate TGF-?1:receptor complex formation: possible consequences for TGF-?1 signaling. Sci Rep. 2017; 7: 1210.
- Rother S, Samsonov SA, Moeller S, Schnabelrauch M, Rademann J, Blaszkiewicz J, Köhling S, Waltenberger J, Pisabarro MT, Scharnweber D, Hintze V. Sulfated hyaluronan alters endothelial cell activation in vitro by controlling the biological activity of the angiogenic factors vascular endothelial growth factor-A and tissue inhibitor of metalloproteinase-3. ACS Appl Mater Interfaces. 2017; 9: 9539-50.
- Lemmnitzer K, Riemer T, Groessl M, Süß R, Knochenmuss R, Schiller J. Comparison of ion mobility-mass spectrometry and pulsed-field gradient nuclear magnetic resonance spectroscopy for the differentiation of chondroitin sulfate isomers. Anal Methods. 2016;8: 8483-91.
- Köhling S, Exner MP, Nojoumi S, Schiller J, Budisa N, Rademann J. One-pot synthesis of anomeric glycosyl thiols from highly functionalized unprotected sugars in water for use in glycan ligation reactions. Angew Chem Int Ed. 2016;55:15510-4.
- Köhling S, Künze G, Lemmnitzer K, Bermudez M, Wolber G, Schiller J, Huster D, Rademann J. Chemoenzymatic synthesis of nonasulfated tetrahyaluronan with a paramagnetic tag for studying its complex with interleukin-10. Chem Eur J. 2016;22:5563-74.
- Nimptsch A, Schiller J. The selected matrix influences the matrix-assisted laser desorption/ionization time-of-flight mass spectral patterns of partially deuterated glycosaminoglycan disaccharides. Rapid Commun Mass Spectrom. 2016; 30: 2164-70.
- Künze G, Köhling S, Vogel A, Rademann J, Huster D. Identification of the glycosaminoglycan binding site of interleukin-10 by NMR spectroscopy. J Biol Chem. 2016;291:3100-13.
- Panitz N, Theisgen S, Samsonov SA, Gehrcke JP, Baumann L, Bellmann-Sickert K, Köhling S, Pisabarro MT, Rademann J, Huster D, Beck-Sickinger AG. The structural investigation of gly-cosaminoglycan binding to CXCL12 displays distinct interaction sites. Glycobiology. 2016; 26(11):1209-1221.
- Rother S, Samsonov S, Hempel U, Vogel S, Möller S, Blaszkiewicz J, Köhling S, Schnabelrauch M, Rademann J, Pisabarro MT, Hintze V, Scharnweber D. Sulfated hyaluronan alters the interaction profile of TIMP-3 with the endocytic receptor LRP-1 cluster II and IV and increas-es the extracellular TIMP-3 level of human bone marrow stromal cells. Biomacromolecules. 2016;17:3252-61.
- Rother S, Samsonov SA, Hofmann T, Köhling S, Blaszkiewicz J, Moeller S, Schnabelrauch M, Rademann J, Kalkhof S, von Bergen M, Pisabarro MT, Scharnweber D, Hintze V. Structural and functional insights into the interaction of sulfated glycosaminoglycans with tissue inhibitor of metalloproteinase-3 – A possible regulatory role on extracellular matrix homeostasis. Acta Biomater. 2016; 45;143-54.
- Hofmann T, Samsonov SA, Pichert A, Lemmnitzer K, Schiller J, Huster D, Pisabarro MT, von Bergen M, Kalkhof S. Structural analysis of the interleukin-8/glycosaminoglycan interactions by amide hydrogen/deuterium exchange mass spectrometry. Methods. 2015; 89:45-53.
- Lemmnitzer K, Schiller J, Becher J, Möller S, Schnabelrauch M. Improvement of the digestibility of sulfated hyaluronans by bovine testicular hyaluronidase: a UV spectroscopic and mass spectrometric study. Biomed Res Int. 2014;2014:986594.
- Penk A, Förster Y, Scheidt HA, Nimptsch A, Hacker MC, Schulz-Siegmund M, Ahnert P, Schiller J, Rammelt S, Huster D. The pore size of PLGA bone implants determines the de novo formation of bone tissue in tibial head defects in rats. Magn Reson Med. 2013; 70:925-35. Schnabelrauch M, Scharnweber D, Schiller J. Sulfated glycosaminoglycans as promising artificial extracellular matrix components to improve the regeneration of tissues. Curr Med Chem. 2013; 20: 2501-23.
- Horatscheck A, Wagner S, Ortwein J, Lisurek M, Kim BG, Beligny S, Schütz A, Rademann J. Benzoylphosphonate-based photoactive phosphopeptide mimetics for functional modulation of protein tyrosine phosphatases and highly specific covalent labeling of SH2-domains, Angew. Chem. 2012, 124, 9577-9583; Angew. Chem. Int. Ed. 2012, 51, 9441-9447.
- Böhme J, Anderegg U, Nimptsch A, Nimptsch K, Hacker M, Schulz-Siegmund M, Huster D, Schiller J. De novo biosynthesis of glycosaminoglycans in the extracellular matrix of skin studied by matrix-assisted laser desorption/ionization mass spectrometry. Anal Biochem. 2012; 421: 791-3.
- Nimptsch K, Süß R, Schnabelrauch M, Nimptsch A, Schiller J. Positive ion MALDI-TOF mass spectra are more suitable than negative ion spectra to characterize sulphated glycosaminoglycans. Int J Mass Spectrom. 2012; 310: 72-6.
- Stübs G, Rupp B, Schiller J, Becher J, Möller S, Nimptsch K, Riemer T, Schnabelrauch M. Synthesis and charac-terization of chemically modified glycosaminoglycans of the extracellular matrix. Mini-Rev Org Chem. 2010; 7: 290-9.
- Schumann RR, Schröder NWJ, Rademann J. Chemoenzymatic synthesis of a glycolipid library and elucidation of the antigenic epitope for construction of a vaccine against Lyme disease. Chem Eur J. 2010; 16: 3536-44.
- Nimptsch K, Süss R, Riemer T, Nimptsch A, Schnabelrauch M, Schiller J. Differently complex oligosaccharides can be easily identified by matrix-assisted laser desorption and ionization time-of-flight mass spectrometry directly from a standard thin-layer chromatography plate. J Chromatogr A. 2010; 1217: 3711-5.
- Weinert S, Jabs S, Supanchart C, Schweizer M, Gimber N, Richter M, Rademann J. Stauber T, Kornak U, Jentsch TJ. Lysosomal Pathology and Osteopetrosis upon Loss of H+-Driven Lysosomal Cl- Accumulation. Science. 2010; 328: 1401-13.
- Schmidt M, El-Dahshan A, Keller S, Rademann J. Selective identification of cooperatively binding fragments in a high-throughput assay enables development of a picomolar caspase-3 inhibitor. Angew Chem Int Ed. 2009; 48: 6346-9.
- Nimptsch A, Schibur S, Schnabelrauch M, Fuchs B, Huster D, Schiller J. Characterization of the quantitative rela-tionship between signal-to-noise (S/N) ratio and sample amount on-target by MALDI-TOF MS: Determination of chondroitin sulfate subsequent to enzymatic digestion. Anal Chim Acta. 2009; 635: 175-82.
- Bauer J, Brandenburg K, Zähringer U, Rademann J. Chemical synthesis of a glycolipid library by a solid phase strategy allows to elucidate the structural specificity of immune stimulation by rhamnolipids. Chem Eur J. 2006; 12: 7116-24.
- Bauer J, Rademann J. Hydrophobically assisted switching phase synthesis: The flexible combination of solid-phase and solution-phase reactions employed for oligosaccharide preparation. J Am Chem Soc. 2005; 127: 7296-7.
- Rademann J, Geyer A, Schmidt RR. Solid-Phase Supported Synthesis of the Branched Pentasaccharide Moiety That Occurs in Most Complex Type N-Glycan Chains. Angew Chem Int Ed Engl. 1998; 37: 1241-5.
- Rademann J, Schmidt RR. Repetitive solid phase glycosylation on an alkyl-thiol polymer leading to sugar oli-gomers containing 1,2-trans- and 1,2-cis-glycosidic linkages. J Org Chem. 1997; 62: 3650-3.
Contact

Prof. Dr. rer.nat. Jörg Rademann
University Professor of Pharmaceutical/Medical Chemistry
Free University of Berlin
Department of Biology, Chemistry, Pharmacy
Institute of Pharmacology
Königin-Luise-Str. 2+4, 14195 Berlin
Phone: +49 (0)30 838 53272
E-Mail: joerg.rademann@fu-berlin.de
Web: bcp.fu-berlin.de/pharmazie

PD Dr. rer.nat. Jürgen Schiller
Research Assistant
Faculty of Medicine of Leipzig University
Institute of Medical Physics and Biophysics
Härtelstraße 16/18, 04107 Leipzig
Phone: +49 (0)341 97-15733
E-Mail: juergen.schiller@medizin.uni-leipzig.de
Web: biophysik.medizin.uni-leipzig.de
