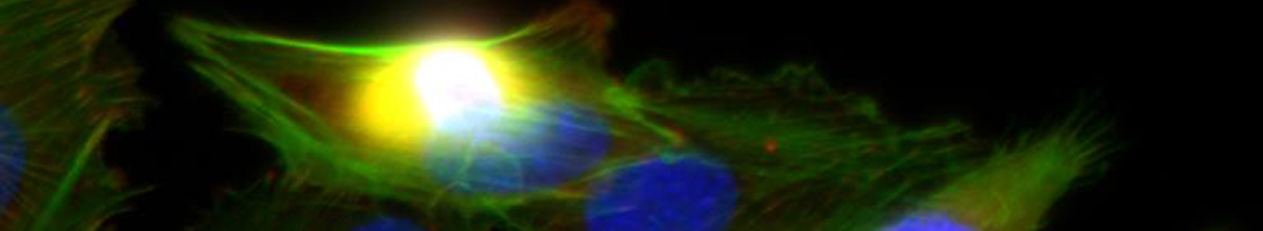
B5 – In vivo- and ex vivo-investigation of the effects of artificial matrices on implant surfaces in long bones
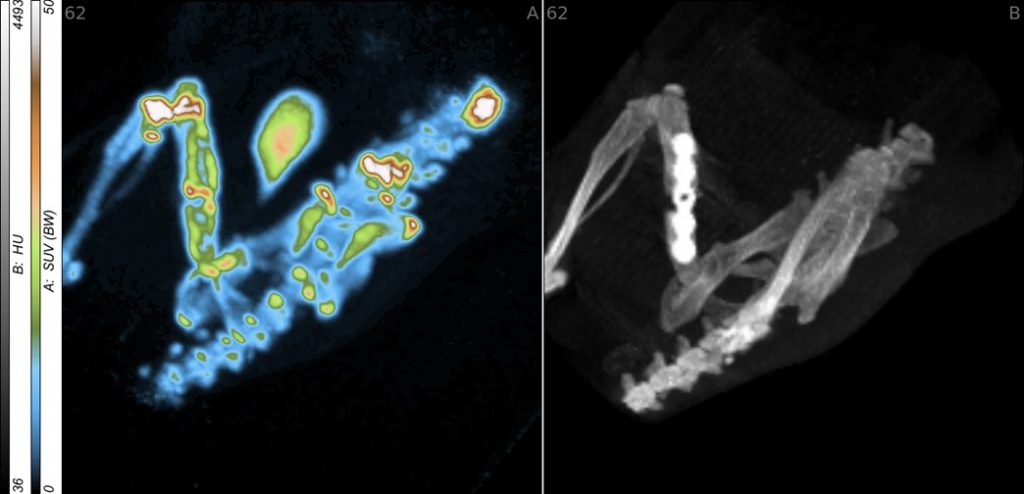
Rapid and stable integration of metallic implants into long bones is aimed at reducing loosening and infection rates and thus an essential factor for outcomes in trauma and reconstructive surgery. For it, a deepened understanding of the healing process is necessary. Therefore, the early stages of wound and bone healing were analysed using microdialysis and the in vitro characterized aECM was investigated in small and large animal models. Based on these results the healing process and metabolic key effects of the microenvironment are to be described by non-invasive, multimodal imaging techniques in vivo. Here established and newly developed radio- and fluorescence tracers as well as a combination of small animal positron emission tomography/computed tomography (PET/CT) and optical imaging (OI) will be used. These approaches allow a functional and partially quantitative characterisation of the whole healing process in dependence upon the used materials. In addition, multimodal imaging will be used to investigate the targeted manipulation of selected inflammatory and angiogenic processes such as inhibition of cyclooxygenase-2 (COX-2), release of nitric oxide (NO), or the activation of the sphingosine-1-phosphate receptor-1 (S1P1).
The results of animal experiments in healthy or diabetic rats suggest that aECM-coated scaffolds have a positive effect on bone healing. In vitro investigations with damaged osteoblast and osteoclasts/peripheral blood mononuclear cells (PBMC) from patients with diabetes mellitus and osteoporosis will be used to prove the clinical relevance of the previous results in a translational approach.
Publications
- Schulze S, Rothe R, Neuber C, Hauser S, Ullrich M, Pietzsch J, Rammelt S. Men who stare at bone: multimodal monitoring of bone healing. Biological Chemistry, vol. 402, no. 11, 2021, pp. 1397-1413. https://doi.org/10.1515/hsz-2021-0170.
- Rothe R, Hauser S, Neuber C, Laube M, Schulze S, Rammelt S, Pietzsch J. Adjuvant Drug-Assisted Bone Healing: Advances and Challenges in Drug Delivery Approaches. Pharmaceutics 2020, 12, 428; doi:10.3390/pharmaceutics12050428.
- Pant K, Neuber C, Zarschler K, Wodtke J, Meister S, Haag R, Pietzsch J, Stephan H. Active Targeting of Dendritic Polyglycerols for Diagnostic Cancer Imaging. Small. 2019 Dec 26. https://doi.org/10.1002/smll.201905013.
- Neuber C, Schulze S, Förster Y, Hofheinz F, Wodke J, Möller S, Schnabelrauch M, Hintze V, Scharnweber D, Rammelt S, Pietzsch J. Biomaterials in repairing rat femoral defects: In vivo insights from small animal positron emission tomography/computed tomography (PET/CT) studies. Clin Hemorheol Microcirc. 2019; 73:177-194. doi: 10.3233/CH-199208.
- Ahlfeld T, Schuster FP, Förster Y, Quade M, Akkineni AR, Rentsch C, Rammelt S, Gelinsky M, Lode A. 3D Plotted Biphasic Bone Scaffolds for Growth Factor Delivery: Biological Characterization In Vitro and In Vivo. Adv Healthc Mater 2019 Mar 6:e1801512. doi: 10.1002/adhm.201801512
- Godoy-Santos AL, Lopes D, Giarola I, de Cesar-Netto C, Rammelt S. Changes in cartilage, synovial cells and synovial fluid after malleolar fractures: What its importance for post-traumatic ankle osteoarthitis? FussSprungg 17: 68-74, 2019
- Laube M, Kniess T, Neuber C, Haase-Kohn C, Pietzsch J. Fluorine-18 Labeling of S100 Proteins for Small Animal Positron Emission Tomography. Methods Mol Biol. 2019; 1929:461-485.
- Rothe R, Schulze S, Neuber C, Hauser S, Rammelt S, Pietzsch J. Adjuvant drug-assisted bone healing: Part I – Modulation of inflammation. Clin Hemorheol Microcirc. 2019 Jun 3. doi: 10.3233/CH-199102
- Rothe R, Schulze S, Neuber C, Hauser S, Rammelt S, Pietzsch J. Adjuvant drug-assisted bone healing: Part II – Modulation of angiogenesis. Clin Hemorheol Microcirc. 2019 Jun 3. doi: 10.3233/CH-199103
- Rothe R, Schulze S, Neuber C, Hauser S, Rammelt S, Pietzsch J. Adjuvant drug-assisted bone healing: Part III – Further strategies for local and systemic modulation. Clin Hemorheol Microcirc. 2019 Jun 3. doi: 10.3233/CH-199104
- Neuber C, Belter B, Meister S, Hofheinz F, Bergmann R, Pietzsch HJ, Pietzsch J. Overexpression of Receptor Tyrosine Kinase EphB4 Triggers Tumor Growth and Hypoxia in A375 Melanoma Xenografts: Insights from Multitracer Small Animal Imaging Experiments. Molecules. 2018 Feb 17;23(2). pii: E444. doi: 10.3390/molecules23020444
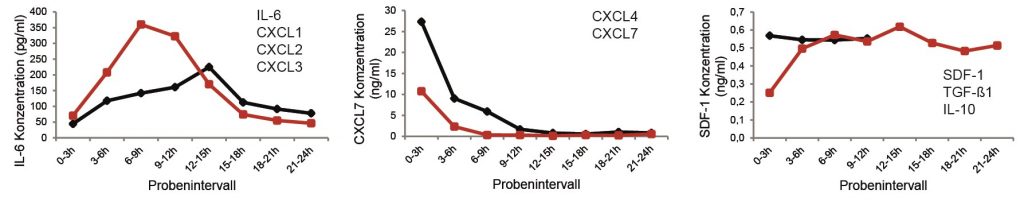
- Förster Y, Schmidt JR, Wissenbach DK, Pfeiffer SEM, Baumann S, Hofbauer LC, von Bergen M, Kalkhof S, Rammelt S. Microdialysis sampling from wound fluids enables quantitative assessment of cytokines, proteins, and metabolites reveals bone defect-specific molecular profiles. PLoS One. 2016;11:e0159580.
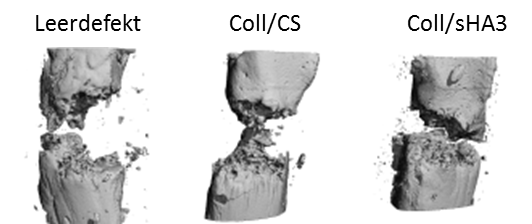
- Förster Y, Bernhard R, Hintze V, Möller S, Scharnweber D, Schnabelrauch M, Rammelt S. Collagen/Glycosaminoglycan coatings enhance new bone formation in a critical size bone defect – a pilot study in rats. Mater Sci Eng C. 2017;71:84-92.
- Picke AK, Salbach-Hirsch J, Hintze V, Rother S, Rauner M, Kascholke C, Möller S, Bernhardt R, Rammelt S, Pisabarro MT, Ruiz-Gómez G, Schnabelrauch M, Schulz-Siegmund M, Hacker MC, Scharnweber D, Hofbauer C, Hofbauer LC. Sulfated hyaluronan improves bone regeneration of diabetic rats by binding sclerostin and enhancing osteoblast function. Biomaterials. 2016;96:11-23.
- Tondera C, Hauser S, Krüger-Genge A, Jung F, Neffe AT, Lendlein A, Klopfleisch R, Steinbach J, Neuber C, Pietzsch J. Gelatin-based hydrogel degradation and tissue interaction in vivo: insights from multimodal preclinical imaging in immunocompetent nude mice. Theranostics. 2016;6:2114-28.
- Laube M, Gassner C, Sharma SK, Günther R, Pigorsch A, König J, Köckerling M, Wuest F, Pietzsch J, Kniess T. Diaryl-substituted (dihydro)pyrrolo[3,2,1-hi]indoles, a class of potent COX-2 inhibitors with tricyclic core structure. J Org Chem. 2015;80:5611-24.
- Kalkhof S, Förster Y, Schmidt J, Schulz MC, Weißflog A, Gao W, Hempel U, Eckelt U, Rammelt S, von Bergen M. Proteomics and metabolomics for in situ monitoring of wound healing. Biomed Res Int. 2014;2014:934848.
- Rentsch C, Rentsch B, Heinemann S, Bernhardt R, Bischoff B, Förster Y, Scharnweber D, Rammelt S. EZM inspired coating of embroidered 3D scaffolds enhances calvaria bone regeneration. Biomed Res Int. 2014;2014:217078.
- Ullm S, Krüger A, Tondera C, Gebauer TP, Neffe AT, Lendlein A, Jung F, Pietzsch J. Biocompatibility and inflammatory response in vitro and in vivo to gelatin-based biomaterials with tailorable elastic properties. Biomaterials. 2014;35:9755-66.
- Dudeck J, Rehberg S, Bernhardt R, Schneiders W, Zierau O, Manjubala I, Goebbels J, Vollmer G, Fratzl P, Scharnweber D, Rammelt S. Increased bone remodelling around titanium implants coated with chondroitin sulfate in ovariectomized rats. Acta Biomater. 2014;10:2855-65.
- Rentsch C, Schneiders W, Hess R, Rentsch B, Bernhardt R, Spekl K, Schneider K, Scharnweber D, Biewener A, Rammelt S. Healing properties of surface coated polycaprolactone-co-lactide scaffolds – a pilot study in sheep. J Biomater Appl. 2014; 28:654-66.
- Förster Y, Gao W, Demmrich A, Hempel U, Hofbauer LC, Rammelt S. Monitoring the first stage of bone healing with microdialysis. Acta Orthop. 2012; 84(1): 76–81.
- Förster Y, Rentsch C, Schneiders W, Bernhardt R, Simon JC, Huster D, Worch H, Rammelt S. Surface modification of implants in long bone. Biomatter. 2012;2(3):149-57.
- Penk A, Förster Y, Scheidt HA, Nimptsch A, Hacker M, Schulz-Siegmund M, Ahnert P, Schiller J, Rammelt S, Huster D. The pore size of PLGA bone implants determines the de novo formation of bone tissue in tibial head defects in rats. Magn Resonan Med. 2013;70(4):925-35.
- Rentsch B, Bernhardt R, Scharnweber D, Schneiders W, Rammelt S, Rentsch C. Embroidered and surface coated polycaprolactone-co-lactide scaffolds: A potential graft for bone tissue engineering. Biomatter. 2012;2:158-65.
- Schneiders W, Rehberg S, Rentsch C, Rein S, Zwipp H, Rammelt S. Effect of chondroitin sulphate on osteogenetic differentiation of human mesenchymal stem cells. Mater Sci Eng C. 2012;32:1926-30.
- Garbe AI, Roscher A, Schüler C, Lutter AH, Glösmann M, Bernhardt R, Chopin M, Hempel U, Hofbauer LC, Rammelt S, Egerbacher M, Erben RG, Jessberger R. Regulation of bone mass and osteoclast function depend on the F-actin modulator SWAP-70. J Bone Miner Res. 2012;27:2085-96.
- Weber F, Böhme J, Scheidt HA, Gründer W, Rammelt S, Hacker M, Schulz-Siegmund M, Huster D. 31P and 13C solid state NMR spectroscopy to study collagen synthesis and biomineralization in polymer-based bone implants. NMR Biomed. 2012;25:464-75.
- Hamann C, Göttsch C, Mettelsiefen J, Henkenjohann V, Rauner M, Hempel U, Bernhardt R, Fratzl-Zelman N, Roschger P, Rammelt S, Günther KP, Hofbauer LC. Delayed bone regeneration and low bone mass in a rat model of insulin-resistanz type 2 diabetes mellitus is due to impaired osteoblast function. Am J Physiol Endocrinol Metab;2011;301:e1220-8.
- Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants – A review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692-709.
- Schneiders W, Reinstorf A, Biewener A, Serra A, Kinscher M, Heineck J, Grass R, Rehberg S, Zwipp H, Rammelt S. In vivo effects of modification of hydroxyapatite/collagen composites with and without chondroitin sulphate on bone remodelling in the sheep tibia. J Orthop Res. 2009;27:15-21.
- Rammelt S, Corbeil D, Manthey S, Zwipp H, Hanisch U. Immunohistochemical in situ characterization of orthopaedic implants on PMMA embedded cutting and grinding sections. J Biomed Mater Res A. 2007;83:313-322.
- Rammelt S, Heck C, Bernhardt R, Bierbaum S, Scharnweber D, Goebbels J, Ziegler J, Biewener A, Zwipp H. In vivo effects of coating loaded and unloaded Ti implants with collagen, chondroitin sulfate and hydroxyapatite in the sheep tibia. J Orthop Res. 2007;25:1052-1061.
- Biewener A, Meyer J, Rentsch C, Grass R, Günther KP, Zwipp H, Rammelt S. Stabilisierung von meta- und diaphysären Segmentdefekten nach Tumorresektion durch Marknagelung und Polymethylmetacrylat- (PMMA-)Formkörper. Orthopäde. 2007;36:152-164.
- Schneiders W, Reinstorf A, Ruhnow M, Rehberg S, Heineck J, Hinterseher I, Biewener A, Zwipp H, Rammelt S. Effect of chondroitin sulphate on material properties and bone remodelling around hydroxyapatite/collagen composites. J Biomat Mater Res A. 2007;85: 638-45.
- Schneiders W, Reinstorf A, Pompe W, Grass R, Biewener A, Holch M, Zwipp H, Rammelt S. Effect of modification of hydroxyapatite/collagen composites with sodium citrate, phosphoserine, phosphoserine/RGD-peptide and calcium carbonate on bone remodelling. Bone. 2007;40:1048-1059.
- Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H, Schneiders W.Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials. 2006;27:5561-5571.
- Rammelt S, Neumann M, Hanisch U, Reinstorf A, Pompe W, Zwipp H, Biewener A. Osteocalcin enhances bone remodeling around hydroxyapatite-collagen composites. J Biomed Mater Res A. 2005;73:284-294.
- Rammelt S, Schulze E, Witt M, Petsch E, Biewener A, Pompe W, Zwipp H.Collagen type I increases bone remodelling around hydroxyapatite implants in the rat tibia. Cells Tissues Organs. 2004;178:146-157.
- Rammelt S, Schulze E, Bernhardt R, Hanisch U, Scharnweber D, Worch H, Zwipp H, Biewener A.Coating of titanium implants with type I collagen. J Orthop Res. 2004;22:1025-1034.
Contact

Prof. Dr. med. Stefan Rammelt
Head of Ankle, Foot and Pediatric Orthopaedics Section
University Hospital “Carl Gustav Carus” of the TUD
Clinic and Polyclinic for Trauma and Reconstructive Surgery
Fetscher Straße 74, 01307 Dresden
Phone: +49 (0)351 458-2208
E-Mail: stefan.rammelt@uniklinikum-dresden.de

Prof. Dr. Jens Pietzsch
Head of department
Department of Radiopharmaceutical and Chemical Biology
Institute for Radiopharmaceutical Cancer Research
Helmholtz Centre Dresden-Rossendorf /
Department of Chemistry and Food Chemistry
Technische Universität Dresden
Phone: +49 (0)351 260 – 2622
E-Mail: j.pietzsch@hzdr.de
Web: www.hzdr.de/db
