B3 – Immun responses to native and artificial extracellular matrices
Superior goal of this project is to characterize artificial extracellular matrices (aECM) which regulate misbalanced activation of immune cells in chronic wounds and which therefore could be applied as immunomodulating component in dressings for advanced wound therapy.
Classically, chronic wounds are characterized by excessive activity of proteases (MMPs, MPO, neutrophil elastase) and reactive oxygen species (ROS) leading to ulceration of the tissue. This high proteolytic environment arises from an unrestrained inflammatory response maintained by an excess of pro-inflammatory cytokines and uncontrolled invasion of immune cells including granulocytes (PMN), monocytes (MO) and macrophages (MA). Persistent activation of inflammatory M1-MA and compromised phenotype switch towards pro-repair M2-MA hindering inflammatory resolution are particularly critical in this respect. Thus, modulating the activity of immune cells and specifically of MA represents a promising approach in the development of advanced wound therapies.
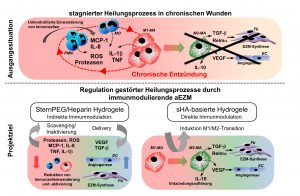
In our previous investigations we have identified aECM based on sulfated glycosaminoglycans (GAG) exerting direct and indirect immunomodulating effects on immune cells. For example, aECM based on collagen I and artificially sulfated hyaluronan (HA) modulate the pro-inflammatory activity of M1-MA and promote the transition towards pro-repair M2-MA. We showed that this direct immunomodulation is mediated by sulfated HA. It involves the recognition and uptake of sulfated HA via CD44 as well as the scavenging receptors CD36 and LOX-1 and implicates the activation of anti-oxidant proteins. M1-MA treated with sulfated HA show despite their pro-inflammatory activation reduced activity of pro-inflammatory transcription factors resulting in attenuated release of pro-inflammatory cytokines in favour of the production of anti-inflammatory mediators. This anti-inflammatory activity of sulfated HA on M1-MA is independent from its molecular weight and can be regulated by adjusting the degree of sulfation of the HA molecule. Furthermore, we identified an indirect immunomodulating effect of hydrogels composed of star-shaped polyethylenglycol (starPEG) and partially desulfated heparins. Immunomodulation by these hydrogels is based on the binding and functional neutralisation (scavenging) of the chemokines MCP-1 and IL-8 within the hydrogel network that is mediated by the heparin component. Due to this inactivation of pro-inflammatory chemokines the hydrogels indirectly modulate the invasion and activation of PMN, MO und MA.
From these results we are deducing our working hypothesis: Resolution of persistent inflammation and promotion of tissue repair in chronic, non-healing wounds can be achieved by multifunctional GAG-based aECM via direct and indirect immunomodulation. Based on our previous research following aspects are currently addressed in subproject B3:
- Analyses of multifunctional and multi-phased starPEG/heparin hydrogels in respect of a multi-step and phase-adapted immunomodulation in chronic wounds
- Development and characterization of multifunctional immunomodulating hydrogels based on sulfated HA
Pro-repair effects of the immunomodulating aECM materials will be analysed in mice models of impaired regeneration based on a metabolic syndrome (db/db) or an induced chronic-venous insufficiency (CVI). Mechanisms of immunomodulation will be characterized in complex co-culture systems with human immune cells and human dermal cells as well as in in vivo models of chronic skin inflammation. Finally, identified immunomodulating mechanisms are validated in a translational approach using wound fluids and immune cells from patients with disturbed wound healing due to diabetes mellitus or CVI.
Publications
- Torregrossa M, Kakpenova A, Simon JC, Franz S. Modulation of macrophage functions by ECM-inspired wound dressings – a promising therapeutic approach for chronic wounds. Biological Chemistry, vol. 402, no. 11, 2021, pp. 1289-1307. https://doi.org/10.1515/hsz-2021-0145.
- Hauck S, Zager P Halfter N, Wandel E, Torregrossa M, Kakpenova A, Rother S, Ordieres M, Räthel S, Berg A, Möller S, Schnabelrauch M, Simon JC, Hintze V, Franz S. Collagen/hyaluronan based hydrogels releasing sulfated hyaluronan improve dermal wound healing in diabetic mice via reducing inflammatory macrophage activity. Bioactive Materials 2021, 6, 4342–4359.DOI: 10.1016/j.bioactmat.2021.04.026.
- Schneider M, Rother S, Möller S, Schnabelrauch M, Scharnweber D, Simon JC, Hintze V, Savkovic V. Sulfated hyaluronan-containing artificial extracellular matrices promote proliferation of keratinocytes and melanotic phenotype of melanocytes from the outer root sheath of hair follicles. J Biomed Mater Res A. 2019 Aug;107(8):1640-1653. doi: 10.1002/jbm.a.36680. Epub 2019 Apr 23.
- Herbert D, Franz S, Popkova Y, Anderegg U, Schiller J, Schwede K, Lorz A, Simon JC, Saalbach A. High fat diet exacerbates early psoriatic skin inflammation independent of obesity: Saturated fatty acids as key players. J Invest Dermatol. 2018; 138:1999-2009.
- Möginger U, Grunewald S, Hennig R, Kuo CW, Schirmeister F, Voth H, Rapp E, Khoo KH, Seeberger P, Simon JC, Kolarich D. Alterations of the human skin N- and O-glycome in basal- and squamous cell carcinoma. Front Oncol. 2018; 8:70.
- Picke AK, Campbell GM, Blüher M, Krügel U, Schmidt FN, Tsourdi E, Winzer M, Rauner M, Vukicevic V, Busse B, Salbach-Hirsch J, Tuckermann JP, Simon JC, Anderegg U, Hofbauer LC, Saalbach A. Thy-1 (CD90) promotes bone formation and protects against obesity. Sci Transl Med. 2018; 10:eaao6806.
- Picke AK, Campbell GM, Schmidt FN, Busse B, Rauner M, Simon JC, Anderegg U, Hofbauer LC, Saalbach A. Thy-1 Deficiency Augments Bone Loss in Obesity by Affecting Bone Formation and Resorption. Front Cell Dev Biol 2018;6:127.
- Schneider M, Lohrenz A, Cross M, Hacker MC, Simon JC, Savkovic V. A human serum-enriched medium formulation supports high viability and marker expression in primary melanocyte cultures from the outer root sheath and epidermis. Exp Dermatol. 2018, 27:87-90.
- Lohmann N, Schirmer L, Atallah P, Wandel E, Ferrer RA, Werner C, Simon JC, Franz S*, Freudenberg U*. Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue wound healing deficiency. Sci Transl Med. 2017;9(386). *equal contribution
- Jouy F, Lohmann N, Wandel E, Ruiz-Gómez G, Pisabarro MT, Beck-Sickinger AG, Schnabelrauch M, Moeller S, Simon JC, Kalkhof S, von Bergen M, Franz S. Sulfated hyaluronan attenuates inflammatory signaling pathways in 1 macrophages involving induction of antioxidants. Proteomics. 2017. [Epub ahead of print]
- Ferrer R, Saalbach A, Grünwedel M, Lohmann N, Forstreuter I, Saupe S, Wandel E, Simon JC, Franz S. Dermal fibroblasts promote alternative macrophage activation improving impaired wound healing. J Invest Dermatol. 2017;137:941-950.
- Friedemann M, Kalbitzer L, Franz S, Moeller S, Schnabelrauch M, Simon JC, Pompe T, Franke K. Instructing human macrophage polarization by stiffness and glycosaminoglycan functionalization in 3D collagen networks. Adv Healthc Mater. 2017;6(7).
- Watarai A*, Schirmer L*, Thönes S*, Freudenberg U, Werner C, Simon JC, Anderegg U. TGFß functionalized starPEG-heparin hydrogels modulate human dermal fibroblast growth and differentiation. Acta Biomater. 2015;25:65-75.
- Scharnweber D, Hübner L, Rother S, Hempel U, Anderegg U, Samsonov SA, Pisabarro MT, Hofbauer L, Schnabelrauch M, Franz S, Simon JC, Hintze V. Glycosaminoglycan derivatives: promising candidates for the design of functional biomaterials. J Mater Sci Mater Med. 2015;26:232.
- Franz S, Muñoz LE, Heyder P, Herrmann M, Schiller M. Unconventional apoptosis of polymorphonuclear neutrophils (PMN): staurosporine delays exposure of phosphatidylserine and prevents phagocytosis by Mo-2 macrophages of PMN. Clin Exp Immunol. 2015;179:75-84.
- Savkovic V, Li H, Seon JK, Hacker M, Franz S, Simon JC. Mesenchymal stem cells in cartilage regeneration. Curr Stem Cell Res Ther. 2014;9(6):469-88
- Anderegg U, Simon JC, Averbeck M. More than just a filler – the role of hyaluronan for skin homeostasis. Exp Dermatol. 2014;23:295-303.
- Kajahn J, Franz S, Rückert E, Forstreuter I, Hintze V, Möller S, Simon JC. Artificial extracellular matrices composed of collagen I and high sulfated hyaluronan modulate monocyte to macrophage differentiation under conditions of sterile inflammation. Biomatter. 2012;2:226-36.
- Franz S, Allenstein F, Kajahn J, Forstreuter I, Hintze V, Möller S, Simon JC. Artificial extracellular matrices composed of collagen I and high-sulfated hyaluronan promote phenotypic and functional modulation of human pro-inflammatory M1 macrophages. Acta Biomater. 2012;9:5621-9.
- Savkovic V, Dieckmann C, Milkova L, Simon JC. Improved method of differentiation, selection and amplification of human melanocytes from the hair follicle cell pool. Exp Dermatol. 2012;21:948-76.
- Förster Y, Rentsch C, Schneiders W, Bernhardt R, Simon JC, Huster D, Worch H, Rammelt S. Surface modification of implants in long bone. Biomatter. 2012;2:149-57.
- Pichert A, Schlorke D, Franz S, Arnhold J. Functional aspects of the interaction between interleukin-8 and sulfated glycosaminoglycans. Biomatter. 2012;2:142-8.
- Salbach J, Rachner TD, Rauner M, Hempel U, Anderegg U, Franz S, Simon JC, Hofbauer LC. Regenerative potential of glycosaminoglycans for skin and bone. J Mol Med. 2012;90:625-35.
- Willenberg A, Saalbach A, Simon JC, Anderegg U. Melanoma cells control HA synthesis in peritumoral fibroblasts via PDGF-AA und PDGF-CC: impact on melanoma cell proliferation. J Invest Dermatol. 2012;132:385-93.
- Van der Smissen A, Hintze V, Scharnweber D, Moeller S, Schnabelrauch M, Majok A, Simon JC, Anderegg U. Growth promoting substrates for human dermal fibroblasts provided by artificial extracellular matrices composed of collagen I and sulfated glycosaminoglycans. Biomaterials. 2011;32:8938-46.
- Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants – A review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692-709.
- Schirmer C, Klein C, von Bergen M, Simon JC, Saalbach A. Human fibroblasts support the expansion of IL-17-producing T cells via up-regulation of IL-23 production by dendritic cells. Blood. 2010;116:1715-25.
- Dieckmann C, Renner R, Milkova L, Simon JC. Regenerative medicine in dermatology: biomaterials, tissue engineering, stem cells, gene transfer and beyond. Exp Dermatol. 2010;19:697-706.
- Saalbach A, Klein C, Sleeman J, Sack U, Kauer F, Gebhardt C, Averbeck M, Anderegg U, Simon JC. Dermal Fibroblasts Induce Maturation of Dendritic Cells. J Immunol. 2007;178:4966-74.
- Averbeck M, Gebhardt C, Voigt S, Beilharz S, Anderegg U, Ch. Termeer, JP Sleeman, Simon JC. Differential Regulation of Hyaluronan Metabolism in the Epidermal and Dermal Compartments of Human Skin by UVB Irradiation. J Invest. Dermatol. 2007;127:687-97.
- Busse K, Averbeck M, Anderegg U, Arnold K, Simon JC, Schiller J. The signal-t-noise ratio as a measure of Hyaluronan oligomer concentration: a MALDI-TOF MS study. Carbohydr Res. 2006;341:1065-70.
- Fieber C, Baumann P, Vallon R, Termeer, Simon JC, Hofmann M, Angel P, Herrlich P, Sleeman JP. Hyaluronan-oligosaccharide-induced transcription of metalloproteases. J Cell Science. 2004;117:359-67.
- Termeer C, Sleeman JP, Simon JC. Hyaluronan – magic glue for the regulation of the immune response? Trends Immunol. 2003;24:112-14.
- Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate Dendritic Cells via the Toll-like Receptor 4. J Exp Med. 2002;195:99-111.
- Weiss JM, Renkl AC, Ahrens T, Moll J, Mai B, Denfeld RW, Schöpf E, Ponta H, Herrlich P, Simon JC. Activation-dependent modulation of hyaluronate receptor expression and hyaluronate avidity by human monocytes. J Invest Dermatol. 1998;111:227-32.
Contact

Prof. Dr. med. Jan-Christoph Simon
Professor of Dermatology, Venerology and Allergology
Faculty of Medicine of Leipzig University
Clinic for Dermatology, Venerology and Dermatology
Philipp-Rosenthal-Straße 23, 04103 Leipzig
Phone: +49 (0)341 97-18600
E-Mail: jan.simon@medizin.uni-leipzig.de
Web: www.uniklinikum-leipzig.de/einrichtungen/dermatologie

PD Dr. rer.nat. Sandra Franz
Group Leader and Research Assistant
Faculty of Medicine of Leipzig University
Clinic for Dermatology, Venerology and Dermatology
Max-Bürger-Forschungszentrum
Johannisallee 30, 04103 Leipzig
Phone: +49 341 97 25880
E-Mail: sandra.franz@medizin.uni-leipzig.de
Web: www.uniklinikum-leipzig.de/einrichtungen/dermatologie
