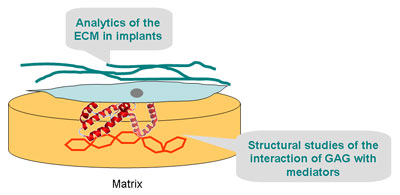
A6 – Investigation of the interaction of mediators with matrix components and analysis of extracellular matrix by NMR
In the third funding period, the investigations of the interaction of regulatory proteins with components of the extracellular matrix will be continued and extended. Our main protein target is sclerostin, which is an important regulator in bone regeneration. Sclerostin is a well-known regulator of the Wnt signaling pathway and intensively studied in project B2. In particular, we are interested in the interaction of sclerostin with GAG.
To study this interaction, we will recombinantly express the protein with NMR active isotopes (15N, 13C, 2H) and subject it to solution NMR studies. Once full NMR signal assignment is achieved, natural and artificial GAGs will be titrated to the protein in order to study its interaction with these molecules. In addition to the experimental determination of structural parameters and binding constants, we shall be carrying out computer simulations to dock the GAGs into the active protein structure in collaboration with A7. The goal of this research is the development of an atomistic model of the complex of sclerostin with various GAGs. As the influence of the Wnt signaling pathway is mediated via the interaction with the protein LRP5/6, we would like to study how GAG can modify the interaction between these proteins. To this end, we will express the first two propeller domains of LRP6 and carry out binding studies with sclersotin in the presence and absence of GAG.
Studies of the diffusion of regulatory proteins in implant materials will also be continued in the next funding period. Finally, we would continue recording the formation of extracellular matrix in bone implants quantitatively. To this end, surface-modified artificial matrices developed by our collaboration partners will be implanted into animal bone models in collaboration with A1, B2, and B5. After explantation, we will carry out the quantitative monitoring of the de novo expression of collagen and bioapatite in the implants by MRI and NMR spectroscopy. We will study samples from a fracture model of the femur of mouse and rat. The incorporation of differently coated materials will be analyzed with respect to the specific chemistry of the surface coat and its influence of the bone healing process.
Publications
- Künze G, Huster D, Samsonov SA. Investigation of the structure of regulatory proteins interacting with glycosaminoglycans by combining NMR spectroscopy and molecular modeling – the beginning of a wonderful friendship. Biological Chemistry, vol. 402, no. 11, 2021, pp. 1337-1355. https://doi.org/10.1515/hsz-2021-0119
- Huster D. Solid-state NMR techniques to study the molecular dynamics in cartilage. In: Xia Y, Momot K. (Eds.): Biophysics and Biochemistry of Cartilage by NMR and MRI. RSC Publishing, Cambridge, 2017, pp. 279-296. (ISBN 978-1-78262-133-1).
- Panitz N, Theisgen S, Samsonov SA, Gehrcke JP, Baumann L, Bellmann-Sickert K, Köhling S, Pisabarro MT, Rademann J, Huster D, Beck-Sickinger AG. The structural investigation of gly-cosaminoglycan binding to CXCL12 displays distinct interaction sites. Glycobiology. 2016; doi: 10.1016/j.actbio.2016.08.030 [Epub ahead of print].
- Hammer N, Huster D, Boldt A, Hädrich C, Koch H, Möbius R, Schulze-Tanzil G, Scheidt HA. A preliminary technical study on sodium dodecyl sulfate-induced changes of the nano-structural and macro-mechanical properties in human iliotibial tract specimens. J Mech Behav Biomed Mater. 2016;61:164-73.
- Köhling S, Künze G, Lemmnitzer K, Bermudez M, Wolber G, Schiller J, Huster D, Rademann J. Chemoenzymatic synthesis of spin-labeled, sulfated tetrahyaluronan for elucidation of its com-plex with interleukin-10. Chemistry. 2016;22:5563-74.
- Künze G, Vogel A, Köhling S, Rademann J, Huster D. Identification of an interleukin-10 gly-cosaminoglycan binding site by NMR spectroscopy. J Biol Chem. 2016; 291:3100-13.
- Nowald C, Penk A, Chiu H, Bein T, Huster D, Lieleg O. A mucin/methylcellulose hybrid gel for applications in wound treatment. Macromol Biosci. 2016;16:567-79.
- Hofmann T, Samsonov SA, Pichert A, Lemmnitzer K, Schiller J, Huster D, Pisabarro MT, von Bergen M, Kalkhof S. Structure analysis of the interleukin-8/glycosaminoglycan complex by amide hydrogen/deuterium exchange mass spectrometry. Methods. 2015;89:45-53.
- Künze G, Gehrcke J-P, Pisabarro MT, Huster D. NMR characterization of the binding proper-ties and conformation of glycosaminoglycans interacting with interleukin-10. Glycobiology. 2014;24:1036-49.
- Samsonov SA, Theisgen S, Riemer T, Huster D, Pisabarro MT. Quantum mechanical, molecu-lar dynamics, and NMR studies of glycosaminoglycans monosaccharide blocks. BioMed Res Int. 2014;2014:808071.
- Künze G, Theisgen S, Huster D. Backbone 1H, 15N, 13C and side chain 13C? NMR chemical shift assignment of murine interleukin-10. Biomol NMR Assign. 2014;8:375-8.
- Kuenze G, Gehrcke JP, Pisabarro MT, Huster D. NMR characterization of the binding properties and conformation of glycosaminoglycans interacting with interleukin-10. Glycobiology, 2014 Jul 6. pii: cwu069. [Epub ahead of print]
- Möbius K, Nordsieck K, Pichert A, Samsonsov SA, Thomas L, Schiller J, Kalkhof S, Pisabarro MT, Beck-Sickinger AG, Huster D. Investigation of lysine side chain interactions of interleukin-8 with heparin and other glycosaminoglycans studied by a methylation-NMR approach. Glycobiology. 2013;23:1260-9.
- Büttner M, Möller S, Keller M, Huster D, Schiller J, Schnabelrauch M, Dieter P, Hempel U. Over-sulfated chondroitin sulfate derivatives induce osteogenic differentiation of human mesenchymal stromal cells independent of bone morphogenetic protein-2 and transforming growth factor-b1 signalling. J Cell Physiology. 2013;228:330-40.
- Förster Y, Scheidt HA, Nimptsch A, Hacker MC, Schulz-Siegmund M, Ahnert P, Schiller J, Rammelt S, Huster D. The pore size of PLGA bone implants determines the de novo formation of bone tissue in tibial head defects in rats. Magn Reson Med. 2012;70:925-35.
- Nordsieck K, Pichert A, Samsonov SA, Thomas L, Berger C, Pisabarro MT, Huster D, Beck-Sickinger AG. Residue 75 of Interleukin-8 is Curicial for the Interaction with Glycosaminoglycans.ChemBioChem. 2012;13:2558-66.
- Penk A, Förster Y, Scheidt HA, Nimptsch A, Hacker M, Schulz-Siegmund M, Ahnert P, Schiller J, Rammelt S, Huster D. The pore size of PLGA bone implants determines the de novo formation of bone tissue in tibial head defects in rats. Magn Resonan Med. 2013; Epub ahead of print.
- Fleddermann J, Pichert A, Arnhold J. Interaction of Serine Proteases from Polymorphonuclear Leucocytes with the Cell Surface and Heparin. Inflammation. 2012;35:81-8.
- Pichert A, Samsonov SA, Theisgen S, Thomas L, Baumann L, Schiller J, Beck-Sickinger AG, Huster D, Pisabarro MT. Characterization of the interaction of the interaction of Interleukin-8 with hyaluronan, chondroitin sulfate, dermatan sulfate, and their sulfated derivatives by spectroscopy and molecular modelling. Glycobiology. 2012;22:134-45.
- Schlorke D, Thomas L, Samsonov S, Huster D, Arnhold J, Pichert A. The Influence of Glycosaminoglycans on IL-8-Mediated Functions of Neutrophils, Carbohydrate Research. 2012;356:196-203. Vlasova II, Sokolov AV, Arnhold J. The free aminon acid tyrosine enhances the chlorinating activity of human myeloperoxidase. J Inorg Chem. 2012;106:76-83.
- Böhme J, Anderegg U, Nimptsch A, Nimptsch K, Hacker M, Schulz-Siegmund M, Huster D, Schiller J. De novo biosynthesis of GAG in the extracellular matrix of skin studied by MALDI MS. Anal Biochem. 2012;421:791-3.
- Weber F, Böhme J, Scheidt HA, Gründer W, Rammelt S, Hacker M, Schulz-Siegmund M, Huster D. 31P and 13C solid state NMR spectroscopy to study collagen synthesis and biomineralization in polymer-based bone implants. NMR Biomed. 2012;25:464-75..
- Flemmig J, Kuchta K, Arnhold J, Rauwald HW. Olea europaea leaf (Ph.eur.) extract as well as several of its isolated phenolics inhibit the gout-related enzyme xanthine oxidase. Phytomedicine. 2011,18:561-66.
- Nimptsch A, Schibur S, Ihling C, Sinz A, Riemer T, Huster D, Schiller J. Quantitative analysis of denatured collagen by collagenase digestion and subsequent MALDI-TOF mass spectrometry. Cell Tissue Res. 2011,343:605-17.
- Yurkova IL, Arnhold J, Fitzl G, Huster D. Fragmentation of mitochondrial cardiolipin by copper ions in the Atp7b-/- mouse model of Wilson’s disease. Chem Phys Lipids. 2011;164:393-400.
- Arnhold J, Flemmig J. Human myeloperoxidase in innate and acquired immunity. Arch Biochem Biophys. 2010;500:92-106.
- Flemmig J, Arnhold J. Interaction of hyperchlorous acid and myeloperoxidase with phosphatidylserine in the presence of ammonium ions. J Inorg Biochem. 2010;104:759-64.
- deAzevedo ER, Ayrosa AM, Faria GC, Cervantes HJ, Huster D, Bonagamba TJ, Pitombo RN, Rabbani SR. The effects ot anticalcification treatments and hydration on the molecular dynamics of bovine pericardium collagen as revealed by 13C solid-state NMR. Magn Reson Chem. 2010;48:704-11.
- Kirchner J, Flemmig J, Furtmüller PG, Obinger C, Arnhold J. (-)-Epicatechin enhances the chlorinating activity of human myeloperoxidase. Arch Biochem Biophys. 2010;495:21-27.
- Nimptsch A, Schibur S, Schnabelrauch M, Fuchs, Huster D, Schiller J. The simple and fast detection of picomole amounts of chondroitin sulphate by MALDI-TOF mass spectrometry subsequent to enzymatic digestion.The signal-to-noise (S/N) ratio as concenetration measure. Anal Chim Acta. 2009;635:175-182.
Contact

Prof. Dr. Daniel Huster
Professor of Medical Biophysics
Faculty of Medicine of Leipzig University
Institute of Medical Physics and Biophysics
Härtelstraße 16 – 18, 04107 Leipzig
Phone: +49 (0)341 97-15701
E-Mail: daniel.huster@medizin.uni-leipzig.de
Web: biophysik.medizin.uni-leipzig.de
