Project Group C: Modulation of Signal Selectivity
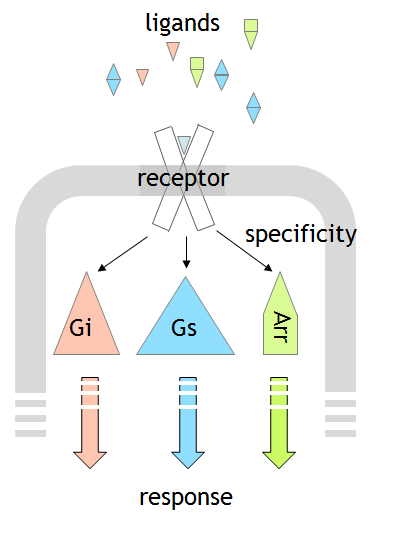
Projects of the group C will study dynamics of GPCR activation with a particular focus on ist localization, activation, effect on transducer coupling, and the consequent spatiotemporal cell signaling pattern. Compartmentation of GPCR signaling demonstrates the complexity of GPCR signaling dynamics. Furthermore, N-terminal versus intracellular signaling will be compared. Fluorescence methods (BRET, FRET, single molecule microscopy) to elucidate the dynamics that yield selective signaling as well as mutational studies will be applied to identify different structural arrangements. The new projects C06, C07 and C08 will perfectly match this focus and contribute by new systems to the overall concept e.g., different generation of the active ligand leads to different cellular responses, or fluorescent cAMP sensors that serve as nanorulers.